
What is the formula of E Enthalpy (or energy) change for a constant-pressure process: E H RTn, where n is the change in the number of moles of gas. The hot gasses (in the form of steam) have to release energy into the environment in the form of heat to cool to the point that they can form liquid water, meaning that the formation of H 2O is exothermic. How do you calculate delta E in chemistry Re: What is delta E E is the change in internal energy of a system. This makes sense - H 2 and O 2 are gasses, while H 2O, the product, is a liquid. Without knowing the specific equation of state (aka, if your gas. However, to determine C P and H you first need an equation of state (such as P V N k T ). If H and C P dont actually depend on pressure, then you can use this equation regardless of whether pressure changes. Since the sign is negative, we know that our reaction is exothermic. Certainly, you agree d H C P d T if were at constant pressure.
-438.png)
For a spontaneous process, the total entropy change, S total is always greater than zero. In our example, our final answer is -13608 J. Where, Gsys Gibbs energy change of the system.Beware strongly exothermic reactions - these can sometimes signify a large release of energy, which, if rapid enough, can cause an explosion. Just write down all the enthalpy changes which make up the two routes, and equate them. The larger the number itself is, the more exo- or endo- thermic the reaction is. On the other hand, if the sign is negative, the reaction is exothermic.
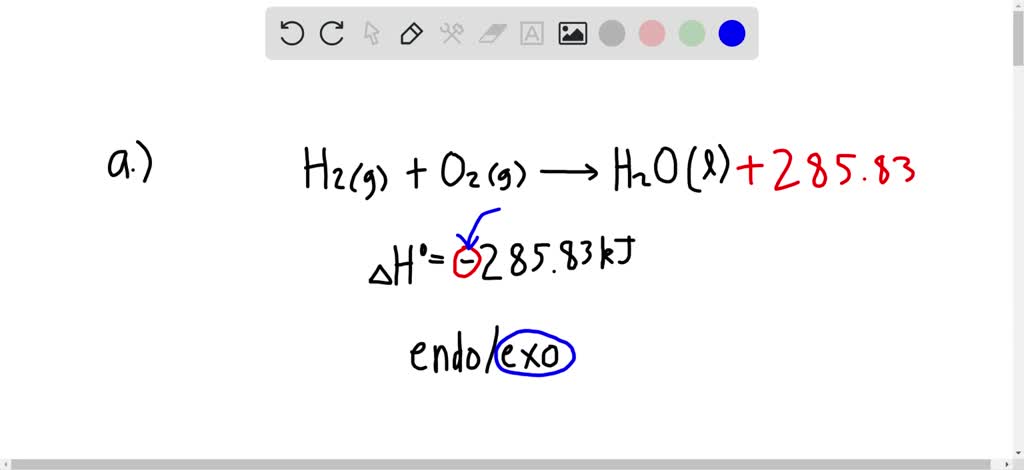
If the sign of your final answer for ∆H is positive, the reaction is endothermic. How do you calculate standard enthalpy change You can use a delta h calculator, a heat of reaction calculator or a. One of the most common reasons that ∆H is calculated for various reactions is to determine whether the reaction is exothermic (loses energy and gives off heat) or endothermic (gains energy and absorbs heat).
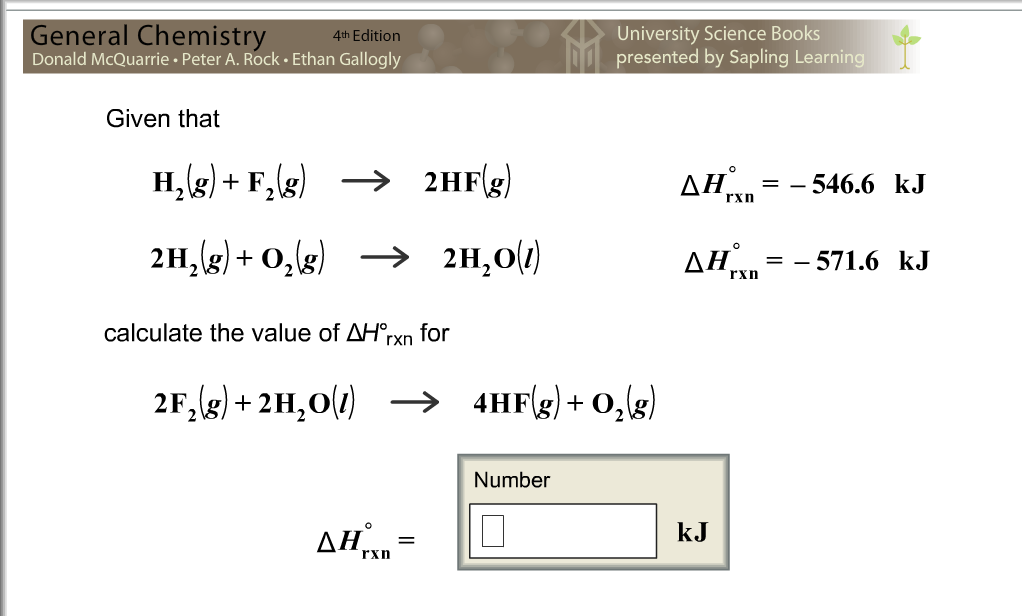
Determine whether your reaction gains or loses energy.


 0 kommentar(er)
0 kommentar(er)
